Abstract
Background: Mantle Cell Lymphoma (MCL) remains incurable, though induction regimens including high-dose cytarabine followed by autologous stem cell transplant (ASCT) provide prolonged disease control. VR-CAP affords superior complete response (CR) rates and survival compared to R-CHOP in older adults with mantle cell lymphoma, but causes significant thrombocytopenia. To improve pretransplant CR rates and outcomes, we administered an alternating regimen of modified VR-CAP and rituximab with high-dose cytarabine (VR-CAP/R+ara-C) on a standard-of-care basis to newly diagnosed MCL patients in need of treatment. Herein we describe our results with this regimen administered at our center, and at referring sites, employing these FDA-approved drugs in a novel sequence prior to ASCT.
Methods: We retrospectively reviewed features of a uniformly treated cohort of patients with newly diagnosed MCL and indications for therapy. VR-CAP was modified to include 2 bortezomib doses (1.3 mg/m2 subcutaneously D1 and D8) and given for 3 cycles, alternating with 3 cycles of rituximab and cytarabine (3 gm/m2 for patients under 60, or 2 gm/m2 for patients 60 or over, 4 doses q12 hours). Other doses were standard, with adjustments left to treating physician discretion.
Cycles were given every 21 days, followed by stem cell collection on recovery from nadir of cycle 6. Growth factor support, biweekly laboratory evaluations, and transfusional support were performed as part of institutional standard-of-care. Events for Kaplan-Meier analysis included disease relapse, refractory disease, start of second line/other therapy, or death. EFS and OS were measured from diagnosis.
Results: 19 patients have been treated to date. Median age is 57 (range 37-67) and 30% are female. MIPI-combined risk group is low: 8, int:6, high: 5.
Patients started VR-CAP/R+ara-C at a median of 28 days from diagnosis (range, 11-106 days). Median number of platelets transfused during VR-CAP/R+ara-C was 4 (range, 0-16), and these occurred exclusively during high-dose cytarabine cycles. Dose reduction of cytarabine was required (clinician discretion) for 6/8 pts starting at 3 gm/m2 and 0/6 pts starting at 2 mg/m2. (For 5 pts. cytarabine dose modifications are unknown).
Complete response to induction (PET, marrow, and endoscopy when indicated) were observed in 14/19 patients, or 14/17 (82%) when considering only the 17 who completed all 6 cycles.
Stem cell collection on recovery from cycle 6 was achieved in 1 day of apheresis for 10 patients; 5 required 2 days. Two patients required plerixafor but others were mobilized with GCSF 10mcg/kg alone. To date, fourteen pts have proceeded to ASCT. Reasons for the 5 others not receiving ASCT include: 2 change of regimen (after bowel perforation complicated the first cycle for one, and due to psychiatric comorbidity for another); 1 required allogeneic transplantation for refractory disease; 1 declined autologous transplant (censored); and 1 has collected but not yet undergone ASCT. Median CD34+ cell dose was 9.5 x 10^6/kg. Median time to platelet engraftment >50k is 13.5 days, and 15 days for neutrophil engraftment >500/uL.
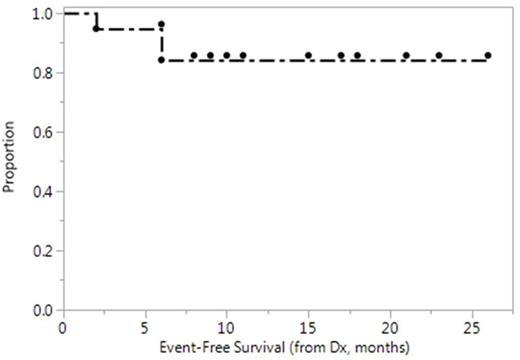
With a median follow-up of 13 months, there have been no deaths among the 19 pts. Rituximab maintenance was given in 6/14 pts who underwent ASCT. EFS is shown in Figure 1. The three observed events include 2 patients who changed therapy as noted and the pt. who had refractory disease and underwent additional salvage and allogeneic SCT.
Conclusions: Alternating induction therapy with VR-CAP/R+ara-C, 3 cycles each, produces a promising CR rate and permits adequate stem cells. Reduction of starting cytarabine dose from 3 gm/m2 among younger MCL patients may reduce platelet transfusion requirements while preserving efficacy of this regimen. Prospective comparative trials of VR-CAP/R+ara-C, and analogous regimens incorporating novel agents, are warranted.
Smith: Sharp: Research Funding; Janssen: Research Funding; Merck: Research Funding; Portola Pharmaceuticals: Research Funding; Dohme Corp: Research Funding; Seattle Genetics: Research Funding; Acerta: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Genentech: Research Funding. Shadman: Genentech: Consultancy, Research Funding; TG Therapeutics: Research Funding; Merck: Research Funding; PLEXXIKON: Research Funding; Emergent: Research Funding; Acerta Pharma: Research Funding; Pharmacyclics: Other: advisory board, Research Funding; Gilead: Research Funding; Celgene: Research Funding; AbbVie: Other: advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal